Description
How it all started?
Patras Medicine was formed in December 2021 with a shared vision; to contribute to the battle against lung cancer. With over 25% of all cancer fatalities attributed to lung cancer, including small and non-small cell lung cancer, it is the deadliest type of cancer among men and women. Detecting lung cancer in its earliest stages, when it is most treatable, is crucial to patients' survival, as with many other cancer types. The cure rate for people with localized, early-stage lung cancer can reach 80% to 90% [1]. The team's objective was to develop a new approach to diagnosis that would be non-invasive, inexpensive, accurate, sensitive, and quick. Shielding and saving human life has been our motivation as Patras Medicine team to develop our test named syn-PNOIA and contribute to the progress of medical science.

Why “syn-PNOIA”?
syn-PNOIA consists of the prefix "syn-," which means "together," and the suffix "-pnoia," which originates from the Greek word "pneo," pointing to breath fresh air. Additionally, without the assistance of Synthetic Biology, or SynBio for short, which gives the prefix "syn" a new meaning, our project would not be possible. With "syn-PNOIA," we breathe safer, healthier, and longer. As a result, collectively, we bring about the symbol of agreement on a healthier way of life.
What about liquid biopsies?
Liquid biopsy allows cancer detection, analysis, and monitoring in various body effluents, such as blood or urine, instead of a fragment of cancer tissue [2]. The body fluid used most commonly for liquid biopsy is blood. By analyzing the sample for cancer cells or circulating tumor DNA, doctors can determine whether a tumor is present or whether treatment is working. In addition, liquid biopsies use blood as a tracking device for neoplastic cells in tumors. They also allow doctors to monitor how treatments work by observing mutations occurring over time within a tumor cell. Liquid biopsies are performed on peripheral blood, which is simple to access, allowing for more widespread use, particularly in patients who cannot undergo surgery.
On the other hand, standard tissue biopsies typically require time-intensive and invasive procedures, such as bronchoscopy for lung cancer, to discover a malignancy. Often, these procedures do not seem appealing to the patient leading to them not getting tested at the onset of the disease. Because of this, liquid biopsies provide a simple way to diagnose malignancies, speeding up tumor identification and treatment of the patient. In addition, liquid biopsy, in contrast to tissue biopsy, allows non-invasive and real-time monitoring of disease development. As the biomarkers have a lifetime of several minutes to a few hours, we can estimate the risk for metastatic relapse or metastatic progression, provide information about the tumor's heterogeneity, and maybe identify therapeutic targets better.
Biomarkers & Circular RNAs
Biomarkers
Biomarkers are tools used to measure the level of risk or predict an outcome of a disease or treatment [3]. They can be used to determine the efficacy of therapy, diagnosis, and prognosis (predicting what will happen next). Biomarkers can be found in blood, urine, saliva, other body fluids, and some organs, and can be DNA, RNA or proteins. Biomarker tests have enabled scientists to locate their presence in correlation with diseases such as cancer and diabetes over the past few decades.
Circular RNAs
After hours of literature review and discussions with professors, our team decided to concentrate on Circular RNAs, a cutting-edge approach to cancer regulation. Circular RNAs (circRNAs) are a class of covalently closed molecules that exert their physiological and pathological effects by acting as protein decoys, protein translators, or microRNA (miRNA) sponges [4] (Fig.1).

They were originally thought to be mere splicing by-products with no functional capacity. Only one circRNA from the sex-determining region Y was found to have any function. In 2013, scientists found the first circRNA that functioned as a miRNA sponge. Today, with the help of RNAseq and Bioinformatics, over 100,000 circRNAs have been identified. Research on circRNA functions, although in its early stages, has shown many findings and is very promising. Specifically, in cancer cells, regulating the expression of many circRNAs seems to have a decisive role in the initiation and progression of the tumor. Numerous studies in the field of cancer [5], including lung cancer [6], [7], have confirmed that circRNAs' abnormal expression contributes to carcinogenesis and the development of malignancies (Fig.2), highlighting their potential as biomarkers for diagnosis, prognosis, and treatment.

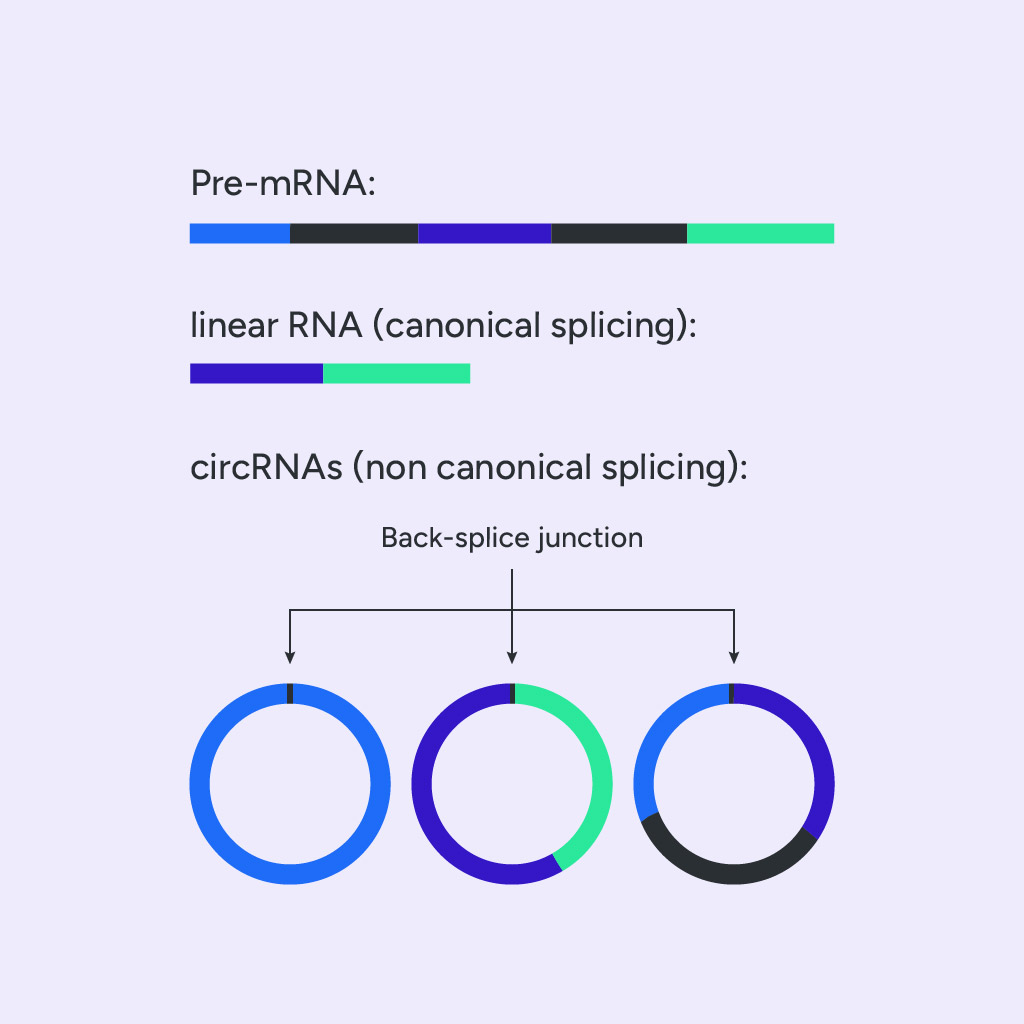
Circular RNAs appear to arise in two ways: either due to competition between linear (canonical) splicing and back-splicing (noncanonical) or as by-products of linear splicing [4]. Back-splicing is a noncanonical form of splicing in which the donor sequence of an upstream exon covalently links to the acceptor sequence of a downstream exon (back-splice junction), which results in the formation of single-stranded circRNA molecules. The back-splice junction site differentiates them from their linear isoforms (Fig.3).
They have the potential to be the ideal biomarkers for Liquid Biopsy. Why?
- The continuous loop structure of circRNAs is covalently closed, which gives them more stability than linear RNAs.
- The detection of circRNAs has mainly been conducted in tissues and cell lines. Nevertheless, they are found in large quantities in human tissues and cells and in body fluids (e.g., blood, saliva, sputum, urine), making them ideal biomarkers for liquid biopsy [8].
- Carcinogenesis and the development of cancer cells may be linked to the abnormal expression of circRNAs in lung cancer.
- Combining circRNAs with traditional cancer biomarkers can confer higher diagnostic accuracy than single traditional biomarkers.
However, research on the pathophysiological functions of circRNAs in lung cancer has a long way to go.
Our biomarkers
We decided it was better to choose a panel of circular RNAs instead of a single one because a panel of biomarkers may improve the predictive performance of the test. The three final targets were hsa_circ_0070354, hsa_circ_0102533, and hsa_circ_0005962.
See the biomarker choice processDNA Nanostructures
General
In principle, constructing detectable DNA nanostructures to respond to disease-specific nucleic acids offers a tool for highly effective biomarker detection in diverse samples [9]. We see the innumerable possibilities that come at a low cost.
Our Design: Linear DNA Nanostructure (LDN)
LDN is a DNA nanostructure that can be used to detect circRNA directly in complex samples, even in cells. The binding of LDN to the target circRNA causes enzyme-free amplification, and then conformational transformations of the two probes occur, emitting fluorescence signal [10].
More specifically, a long linear DNA scaffold with multiple hairpin probe installation sites is synthesized by Rolling Circle Amplification (RCA). The probes (H1 and H2) are then installed on the DNA scaffold because of their complementary hanging tail to form the LDN (Fig.4a).
In the reaction mixture, H1 identifies the unique Backsplice Junction site of circRNA, which is, as mentioned, a diagnostic advantage for distinguishing circRNA from its linear isoforms in a complex sample. H2 contains a pair of fluorophore and quencher groups. In the absence of a target circRNA, no interaction is observed between H1 and H2, which are in a hairpin structure. However, in the presence of the target circRNA, the circRNA hybridizes to H1, which then interacts and hybridizes with H2. During the second hybridization, the fluorophore group dissociates from the quencher and fluorescence is emitted. The released target circRNA can then continue to hybridize with other H1 probes, triggering a non-enzymatic amplification reaction along the linear scaffold, and the entire LDN immediately fluoresces (Fig4b). With the aid of modeling, we designed the probe sequences to hybridize with the three corresponding circRNAs.

Compared to traditional circRNA detection approaches, the LDN-based method presents several advantages:
- It does not require expensive reagents. After LDN is formed, it can perform enzyme-free detection. It is an isothermal process and, unlike PCR, does not require a thermal cycler.
- It can accurately recognize circRNA without interference from its linear isoforms. Therefore, no RNAse treatment is required before the reaction.
- Biomarker detection happens in only 70 min, using a total RNA extract from a potential patient’s blood sample.
Next Steps
- We also propose applying our methodology to visualize intracellular circRNA in situ. Certain circRNAs with prognostic value are not secreted into the circulation. Therefore, their analysis can be a crucial point in obtaining a clearer picture of the state of the tumor. With electroporation, LDN can be inserted in cells derived from a tissue biopsy. With confocal fluorescence microscopy, images can be obtained, analyzing whether or not the circRNA target is present. Testing that aspect of our project was not possible due to time limitations, but trying to implement the same technique for both the prognosis and diagnosis of lung cancer undoubtedly will improve the efficiency of lung cancer treatment.
- In order to validate both our method and our biomarker selection, testing our diagnostic tool in early-stage lung cancer patients' blood samples is essential. Complementary experiments such as RNA-seq to analyze the expression profile of hsa_circ_0070354, hsa_circ_0102533, and hsa_circ_0005962 and other circular RNAs will determine if these novel biomarkers can be readily used for lung detection or if the various lung cancer subtypes differentiate their expression. Cross-validating these results with the ones obtained from patients tested with the LDN system will prove the sensitivity and specificity of our method. However, a large number of patients is necessary to obtain high statistical power data, indicating that clinical trials are in order.
- Implementing the LDN system for diagnosing various other diseases and cancer types through our Modeling work proves the versatility of our design. Just by inputting the mature sequence of a new circRNA target, all the possible LDN structures can be obtained using our simple ldn_generator.py Python script. Furthermore, the same protocol can be applied using isothermal target detection. For more information about the creation and usage of the ldn_generator.py as well as the detection protocol, please refer to the Contribution and Experiments pages, respectively.
- Our team aspires to create a start-up company with the brand name syn-PNOIA. Distribution of our kit to health clinics and microbiological laboratories and analysis of patient samples in-house will provide a multifaceted approach to lung cancer diagnosis. Please refer to the Entrepreneurship page for more information about our next financial steps.
-
[1]. Lung Cancer Statistics | How Common is Lung Cancer? (n.d.). Retrieved October 5, 2022, from https://www.cancer.org/cancer/lung-cancer/about/key-statistics.html
[2]. Poulet, G., Massias, J., & Taly, V. (2019). Liquid Biopsy: General Concepts. Acta Cytologica, 63(6), 449–455. https://doi.org/10.1159/000499337
[3]. Strimbu, K., & Tavel, J. A. (2010, November). What are biomarkers? Current Opinion in HIV and AIDS, 5(6), 463–466. https://doi.org/10.1097/coh.0b013e32833ed177
[4]. Kristensen, L. S., Andersen, M. S., Stagsted, L. V. W., Ebbesen, K. K., Hansen, T. B., & Kjems, J. (2019, August 8). The biogenesis, biology and characterization of circular RNAs. Nature Reviews Genetics, 20(11), 675–691. https://doi.org/10.1038/s41576-019-0158-7
[5]. Chen, L., & Shan, G. (2021, May). CircRNA in cancer: Fundamental mechanism and clinical potential. Cancer Letters, 505, 49–57. https://doi.org/10.1016/j.canlet.2021.02.004
[6]. Di, X., Jin, X., Li, R., Zhao, M., & Wang, K. (2019, March). CircRNAs and lung cancer: Biomarkers and master regulators. Life Sciences, 220, 177–185. https://doi.org/10.1016/j.lfs.2019.01.055
[7]. Wang, C., Tan, S., Li, J., Liu, W. R., Peng, Y., & Li, W. (2020, November). CircRNAs in lung cancer - Biogenesis, function and clinical implication. Cancer Letters, 492, 106–115. https://doi.org/10.1016/j.canlet.2020.08.013
[8]. Wang, S., Zhang, K., Tan, S., Xin, J., Yuan, Q., Xu, H., Xu, X., Liang, Q., Christiani, D. C., Wang, M., Liu, L., & Du, M. (2021, January 11). Circular RNAs in body fluids as cancer biomarkers: the new frontier of liquid biopsies. Molecular Cancer, 20(1). https://doi.org/10.1186/s12943-020-01298-z
[9]. DNA Nanotechnology Tools: From Design to Applications. (2022, April 22). Wyss Institute. Retrieved October 5, 2022, from https://wyss.harvard.edu/technology/dna-nanotechnology-tools-from-design-to-applications/
[10]. Jiao, J., Xiang, Y., Duan, C., Liu, Y., Li, C., & Li, G. (2020, August 24). Lighting Up CircRNA Using a Linear DNA Nanostructure. Analytical Chemistry, 92(18), 12394–12399. https://doi.org/10.1021/acs.analchem.0c02146
