Implementation
Introduction
syn-PNOIA kit will introduce a new era regarding early-stage lung cancer detection, making the diagnosis more straightforward, more accessible, more affordable, and user-friendly for any customer and patient, and significantly increasing patients’ chances of survival. We performed questionnaires, market and financial analyses to implement the product in the real world. By investigating the market, we defined our customers and our goals. The confirmation results of our surveys in the initial phase of Primary Market Research among doctors make us quite hopeful of our product’s potential establishment and success. You can find all of the above in our Business Plan.
Market
People at a high risk of getting lung cancer, such as long-term smokers over 40 years old or employees exposed to asbestos and radium, are the recommended end users. Nonetheless, anyone can incorporate our test in their yearly/6-month check-up. Since it needs trained staff and specialized equipment, our diagnostic tool can be used in hospitals, clinics, and diagnostic centers. The goal for the first years is to approach the Greek market and expand the network worldwide. As shown in the graphics below, we can confirm the acceptance and the need for a new faster method of diagnosing lung cancer in its early stages. More than 100 medical students answered the questionnaire, with more than 50% considering the development of a new diagnostic method a necessity. This generation of doctors is young, innovative, and—with guidance—poised to take on healthcare's current and future challenges. The bar chart displayed below is on a scale of one to five with five indicating the strong agreement of the participants to the particular statement.
Application
The kit's use is simple and about a three step-process after blood sampling. First, with the use of a RNA extraction kit, total RNA is extracted, then added directly to the reaction mixture containing the Linear DNA Nanostructure and the buffer solution. Positive and Negative calibration samples are prepared as well to ensure that the delivered Nanostructure has retained its integrity and purity. After incubation for 70 minutes in a standard thermal cycler the medical personnel can proceed to sample analysis. The fluorescence signal emitted by the fluorescent labels present in the Nanostructure is detected using a Plate-Reader spectrophotometer. The steps are presented in the graphic below and detailed instructions can be found on the corresponding protocol listed in the Product Sheet Proposition listed in section Product Design.
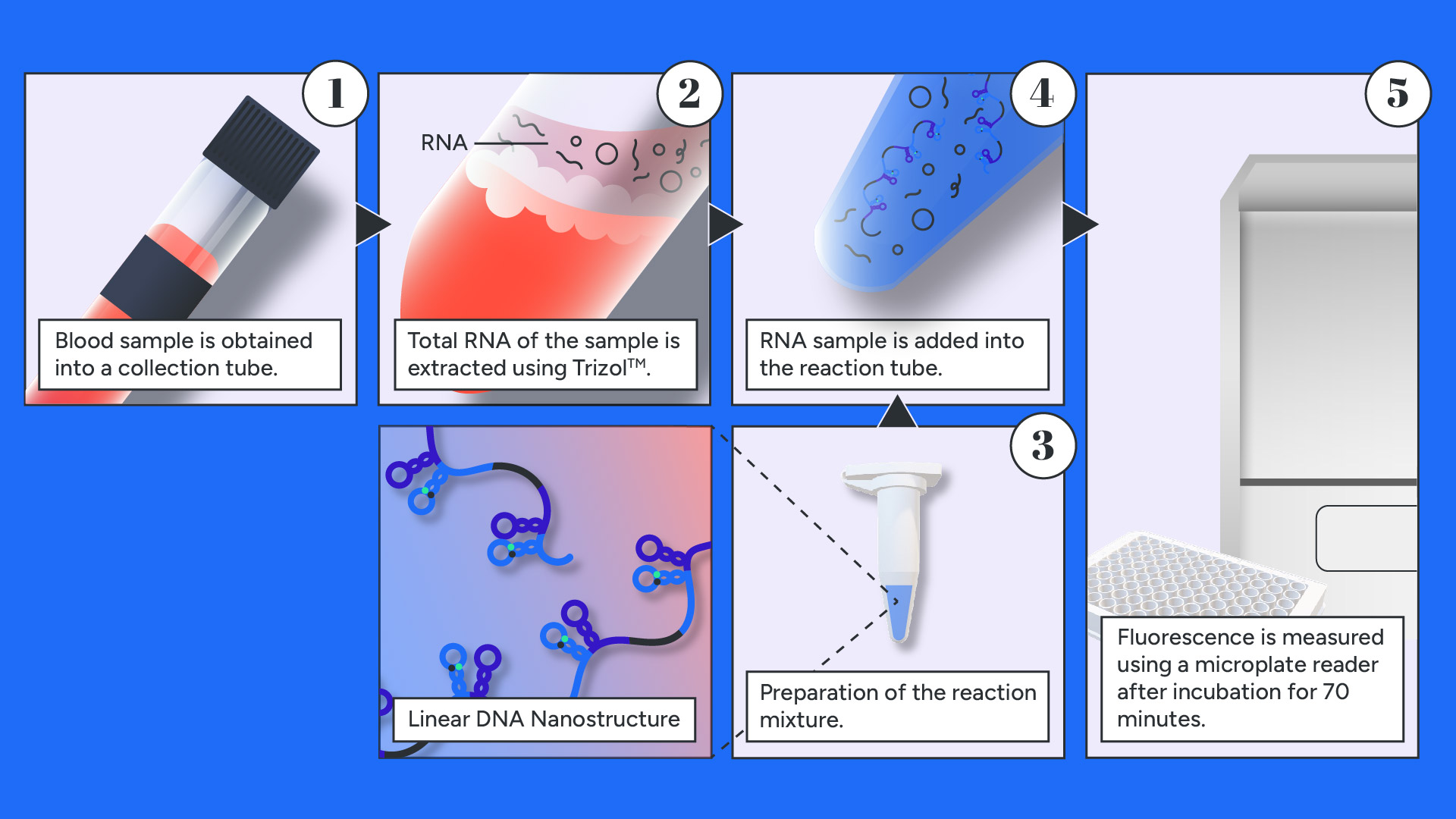
Product Design
Our kit is contained in a sealed foam box to avoid photobleaching and maintain its temperature at -20 ±5 ℃. Inside the box, a 96-well plate is supplied alongside the following reagents:
- Invitrogen™ TRIzol™
- 2nd reagent for TRIzol™
- 1x 200 μL tube of PBS Buffer with 1% BSA (pH=7,4)
- 4x lyophilized Positive Calibration Sample
- 4x lyophilized Negative Calibration Sample
- 12x lyophilized Reaction mixture, containing the Nanostructure
We propose a Product Sheet Proposition that contains detailed instructions and safety precautions of our test.
View final detailed protocolOn top of this, we have produced a graphic movie that walks viewers through the steps required for using the device correctly. In this way, we seek to win the confidence of potential partners, clients, and investors.
Our product design was based on Dr. Klapa’s advice. Dr. Klapa suggested that our diagnostic kit should contain positive and negative calibration samples. When a product is stored for long periods, the fluorescence signal might decay, or our Nanostructure might denature. So, we should include controls that can be tested simultaneously as our sample. In addition, the reference samples must return an expected value to ensure the test is valid. She also suggested that the test should include the total RNA extraction kit besides our diagnostic kit to ensure the ease of testing.
Read the full interview.Can the Nanostructure be successfully shipped from the point of manufacturing to the point of testing?
We may be able to synthesize our product, but can we successfully distribute it to clinics, hospitals, and microbiological laboratories? We have experimentally proven that we are not able to store our Nanostructure in liquid form. For more information please refer to the “LDN Stability” subsection of our Results page.
>We propose the lyophilization of our Nanostructure to preserve its structural integrity during long-term storage. Lyophilization, or freeze drying, enhances the long-term storage stability of cells, proteins, microorganisms, or nanoparticles. Pharmaceutical companies are using freeze drying to increase the shelf life of products, especially at room temperature. However, DNA integrity is said to be compromised by freeze drying due to its vulnerability to common factors, such as pH or ionic interactions. DNA nanostructures can be lyophilized with the addition of stabilizers such as lipids or polymers. For example, plasmid DNA nanoparticles can be lyophilized with high amounts of sugars, such as sucrose or trehalose, in concentrations up to 0.5 M to maintain particle size and transfection activity [1],[2]. Also, DNA/polymer complexes with polymers like poly(2-dimethylamino)ethyl methacrylate are used in the lyophilization of plasmid DNA. This DNA complex, with the addition of sucrose, can maintain its size and integrity for up to 10 months at 4 oC [3].
Furthermore, a study has shown that DNA nanostructures can preserve their structural integrity during freeze-drying with different concentrations of Mg+2. Tetrahedral DNAs and triangular origami DNA nanostructures were frozen with a solution containing 50 μM Mg+2 at -80 oC and then reconstituted with deionized water. Gel electrophoresis demonstrated that DNA molecules had identical mobilities with freshly prepared DNA molecules, and no structural changes were observed [4]. Therefore, DNA nanostructures can easily withstand lyophilization.
Fluorophores stability considerations
Photobleaching is a photochemical alteration of a fluorophore molecule, rendering it unable to fluoresce. It is caused by cleaving covalent bonds or non-specific reactions between the fluorophore and surrounding molecules. The number of excitation cycles to achieve complete bleaching varies. ATTO fluorophores have an incredibly high photostability and high fluorescent quantum yield. However, To ensure optimum activity, fluorescent probes should always be protected from light to avoid photobleaching. That is why we are proposing a sealed foam box for our kit.
Although labeled probes should, for the most part, be handled as other custom oligonucleotide sequences, we recommend resuspending the reagents in PBS buffer (Phosphate-Buffered Saline, pH 7.4). It will not only ensure the optimal reaction conditions but also establish fluorophore stability to avoid decay reactions.
Safety
COVID-19 Considerations
The laboratories that represent the project perform site- and activity-specific risk assessments to determine the most appropriate safety measures to implement for particular circumstances and activities. The staff is up to date with their vaccines, wears face masks and is judicious with personal hygiene and disinfection.
Product Sheet Proposition
We have developed a product sheet with all the details required for using the kit correctly and safely. On top of this, we have produced a graphic movie that walks viewers through the steps required for using the device correctly. In this way, we seek to win the confidence of potential partners, clients, and investors.
Please refer to the previous section to obtain the full Product Sheet Proposition.
Product Sheet Proposition
We have developed a Product Sheet proposition with all the details required for using the kit correctly and safely. We include precaution measures regarding blood sampling contraindications and complications, decontamination of surfaces, and protection procedures.
Other Actions
We've been learning a lot about biosafety in our Values and Risks iGEM workshop. One of the things we learned is that it's important to keep track of who has access to the lab and when they have access, and what they're doing there. We also gained an understanding about the difference between levels of biosafety, how to use different kinds of equipment in your labs, what procedures we should follow if there's an emergency, and more!
Our team is working hard to make sure all our employees are trained on these topics so that they know what they need to do when they work with dangerous materials.
Challenges & Risks
Despite the fact that the product is quite promising, we need to take into consideration possible risks and challenges. Both of them are explained in our Business plan in the SWOT and risk analysis which are presented below.

The weaknesses and threats should not discourage us but make us work harder to overcome those challenges and improve our product. In the graphic below we present the possible risks. Nonetheless, with the right precautions there should be no problem while using our kit.

-
[1] T.J. Anchordoquy, J.F. Carpenter, D.J. Kroll, Maintenance of Transfection Rates and Physical Characterization of Lipid/DNA Complexes after Freeze-Drying and Rehydration, Arch. Biochem. Biophys., 348 (1997) 199-206.
[2] K.K. Abla, M.M. Mehanna, Freeze-drying: A Flourishing Strategy to Fabricate Stable Pharmaceutical and Biological Products, International Journal of Pharmaceutics (2022), doi: https://doi.org/10.1016/j.ijpharm.2022.122233
[3] J.-Y. Cherng, P. van de Wetering, H. Talsma, D.J.A. Crommelin, W.E. Hennink, Freeze-Drying of Poly((2-dimethylamino)ethyl Methacrylate)-Based Gene Delivery Systems, Pharm.Res., 14 (1997) 1838-1841.
[4] Zhu, B., Zhao, Y., Dai, J., Wang, J., Xing, S., Guo, L., … Wang, L. (2017). Preservation of DNA Nanostructure Carriers: Effects of Freeze–Thawing and Ionic Strength during Lyophilization and Storage. ACS Applied Materials & Interfaces, 9(22), 18434–18439. doi:10.1021/acsami.7b04784


